Heat And Temperature
Throughout my career, I noticed that even college students have yet to have a clear idea about temperature and heat. Sometimes, they misunderstand both are the same. In this article, I discuss the difference between heat and temperature.
The following table gives the difference point-wise.
No | Heat | Temperature |
1 | Heat is a form of energy. | Temperature is a measure of that energy |
2 | Heat travels from one object to another due to a temperature difference. | The temperature rises when heated and falls when cooled. |
3 | Able to do work. | Not able to do work |
4 | Units: Joules, Calories, BTU (British Thermal Units), kW (kilowatt) | Kelvin, Celsius, Fahrenheit, Rankine |
5 | Measuring device: Calorimeter | Thermometer |
6 | Symbol: Q | Symbol: T |
7 | The energy of the matter = zero. | Absolute zero |
8 | Heat depends: on speed, size, and number of particles | Temperature is independent of these factors |
9 | Values: positive. | Values: positive and negative |
Heat is the thermal energy transferred between systems due to a temperature difference. [17] In colloquial use, heat sometimes refers to thermal energy itself.
- Thermal energy is the kinetic energy of vibrating and colliding atoms in a substance.
Consider a metal bar conducting heat from the hot end to the cold end.
- If the metal bar is considered a thermodynamic system, the energy flowing within the metal bar is called internal energy, not heat.
- The hot metal bar transfers heat to its surroundings, a correct statement for both the strict and loose meanings of heat.
Heat is energy transfer to or from a thermodynamic system by a mechanism that the microscopic atomic modes of motion or the corresponding macroscopic properties. [18] This descriptive characterization excludes the transfer of energy through thermodynamic work or mass. Defined quantitatively, the heat involved in a process is the difference in internal energy between the final and initial states minus work done in the process. [19] It is the formulation of the first law of thermodynamics.
Calorimetry is a measurement of the quantity of energy transferred as heat by its effect on the states of interacting bodies, for example, by the amount of ice melted or by a change in the temperature of a body. [20]
In the International System of Units (SI), the unit of measurement for heat, as a form of energy, is the joule (J).
Temperature
- Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness.
- We can measure the temperature with a thermometer.
- It reflects the average kinetic energy of vibrating and colliding atoms making up a substance.
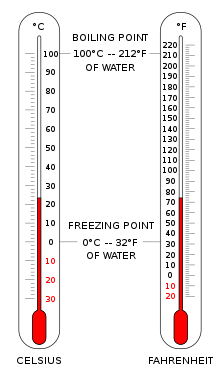
The most common scales are the Celsius scale with the unit symbol °C (formerly called centigrade), the Fahrenheit scale (°F), and the Kelvin scale (K), the latter being used predominantly for scientific purposes. The kelvin is one of the seven base units- in the International System of Units (SI).
- Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale.
- It would be impossible to extract energy as heat from a body at that temperature.
Temperature is significant in all fields of natural sciences, including physics, chemistry, Earth science, astronomy, medicine, biology, ecology, material science, metallurgy, mechanical engineering, geography, and most aspects of daily life. [1]
Effects of temperature
Many physical processes are related to temperature.
Some of them are:
- The physical properties of materials include the phase (solid, liquid, gaseous, or plasma), density, solubility, vapor pressure, electrical conductivity, hardness, wear resistance, thermal conductivity, corrosion resistance, and strength.
- The rate and extent to which chemical reactions occur[21].
- Thermal radiation, properties and amount of radiation emitted from the surface of an object.
- Air temperature affects all living organisms.
- The speed of sound, which in a gas is proportional to the square root of the absolute temperature[22]
Absolute zero
- At this temperature, matter contains no macroscopic thermal energy.
- It has quantum-mechanical zero-point energy as predicted by the uncertainty principle, although this does not enter into the definition of absolute temperature.
- Theoretically, in a body at a temperature of absolute zero, all classical motion of its particles has ceased, and they are at complete rest in this classical sense. Absolute zero, defined as 0 K, is −273.15 °C, or −459.67 °F.
Negative temperature
On the empirical temperature scales, it has no reference to absolute zero. A negative temperature is one below the zero point of the scale used. For example, dry ice has a sublimation temperature of −78.5 °C is equivalent to −109.3 °F.[24] On the absolute Kelvin scale, this temperature is 194.6 K. No body can be brought to 0 K (the temperature of the ideally coldest possible body) by any finite practicable process; this is a consequence of the third law of thermodynamics. [25][26][27]
The internal kinetic theory temperature of a body cannot take negative values. The thermodynamic temperature scale, however, is not so constrained.
For a body of matter, we can define it in terms of microscopic degrees of freedom, namely particle spins, a subsystem with a temperature other than that of the whole body. When the body is in its state of internal thermodynamic equilibrium, the temperature of the body and the subsystem must be the same. The two temperatures can differ when working through externally imposed force fields. The energy transfer occurs to and from the subsystem, separately from the rest of the body. Then, the whole body is not in its state of internal thermodynamic equilibrium. There is an upper limit of energy such a spin subsystem can attain.
Considering the subsystem to be in a temporary state of virtual thermodynamic equilibrium, the possibility is there to obtain a negative temperature on the thermodynamic scale. Thermodynamic temperature is the inverse of the derivative of the subsystem's entropy concerning its internal energy. As the subsystem's internal energy increases, the entropy increases for some range but eventually attains a maximum value and then begins to decrease as the highest energy state begins to fill. At the point of maximum entropy, the temperature function shows the behavior of a singularity because the slope of the entropy as a function of energy decreases to zero and then turns negative. As the entropy of the subsystem reaches maximum value, its thermodynamic temperature goes to positive infinity, switching to negative as the slope turns negative. Such negative temperatures are hotter than any temperatures. Over time, when we expose the subsystem to the rest of the body, which has a higher temperature, energy transfer occurs as heat from the negative temperature subsystem to the positive temperature system. [28] Not defined the kinetic theory temperature for such subsystems.
Transfer of heat:
Conduction:
Referring to conduction, Partington writes: If a hot body is in conducting contact with a cold body, the temperature of the hot body falls, and that of the cold body rises, and a quantity of heat has passed from the hot body to the cold body.[13]
- Conduction is when heat transfer occurs between molecules in direct contact with each other without the movement of the particles.
Why do the tiles feel colder than the mat?
The tiles feel colder than the mat at room temperature.
- Tile is a better conductor than a mat.
- Tiles rapidly take the heat away from the body, making the tile feel colder to the touch than the mat.
- Convection is the movement of fluid molecules from a high-temperature region to a low-temperature.
- As temperature increases, the volume of liquid also increases.
Latent heat of vaporization:
We can supply energy to a substance by heating it. Energy supply leads to a rise in temperature because of an increase in the kinetic energy of the molecules. That is extra energy for the molecules to move around at high speed. If we heat beyond its boiling point, a phase change occurs. For example, if we heat water beyond boiling point (100oC), the water molecules leave the surface as vapors.
- The extra energy results in vaporization and is known as the latent heat of vaporization.
- The added energy does not increase temperature.
- It is a reversible process.
- When the vapors condense into liquid, it will release the absorbed energy.
- Latent heat is also absorbed when a solid melts and released when it freezes.
Following changes occur when we heat an object.
- Energy supply leads to an increase in the kinetic energy of the molecules.
- The speed of motion of molecules increases
- If we heat beyond its boiling point, a phase change occurs
- It is a reversible process
Radiation:
Maxwell writes: In Radiation, the hotter body loses heat, and the colder body receives heat, a process occurs in some intervening medium that does not become hot.[13]
Thermal radiation is known as radiant heat. Thermal radiation is the emission of electromagnetic radiation.
- Radiation emission happens through a vacuum or transparent medium that can be solid or liquid.
- The movement of charged electrons and protons is responsible for the emission of electromagnetic radiation.
- All objects give out energy as infrared rays, similar to X-rays.
- A light bulb gives out a lot of infrared rays.
- These rays will heat anything that absorbs this radiation.
- A dull surface absorbs well, while a smooth surface reflects them.
- Infrared rays are invisible, but we can feel the effect.
For example, If you keep your hand closer to a light bulb, it gets warmer.
The radiation is intense near the bulb.
- Objects give out energy as infrared rays. A light bulb gives out a lot of infrared rays.
- Infrared rays are invisible
- We can feel the effect of infrared rays.
Heat engine
In classical thermodynamics, a commonly considered model is the heat engine. It consists of four bodies: the working body, the hot reservoir, the cold reservoir, and the work reservoir. A cyclic process leaves the working body in the same state and repeats indefinitely. Work transfers between the working body and the work reservoir are reversible, and only one work reservoir is needed. Requires two thermal reservoirs because the transfer of energy as heat is irreversible. A single cycle sees energy taken by the working body from the hot reservoir and sent to the two other reservoirs, the work reservoir and the cold reservoir.
- The hot reservoir supplies energy and the cold reservoir always receives energy.
- The second law of thermodynamics is that no cycle can occur in which no energy transfers to the cold reservoir.
- Heat engines achieve higher efficiency when the ratio of the initial and final temperatures is significant. [1]
Another commonly considered model is the heat pump or refrigerator. Again, there are four bodies: the working body, the hot reservoir, the cold reservoir, and the work reservoir. A single cycle starts with the working body colder than the cold reservoir, and then energy is taken in as heat by the working body from the cold reservoir. Then the work reservoir does work on the working body, adding more to its internal energy, making it hotter than the hot reservoir. The hot working body passes heat to the hot reservoir but remains hotter than the cold reservoir. Then, by allowing it to expand without passing heat to another body, the working body is made colder than the cold reservoir. It can now accept heat transfer from the cold reservoir to start another cycle.
Accordingly, the cycle is still in accord with the second law of thermodynamics.
- The 'efficiency' of a heat pump (which exceeds unity) is best when the temperature difference between the hot and cold reservoirs is least. [1]
Hotness
The property of hotness is a concern of thermodynamics that we can define without reference to the heat. Consideration of hotness leads to the concept of empirical temperature. [2][3] All physical systems are capable of heating or cooling others. [4] Concerning hotness, the definition of the comparative terms hotter and colder is by the rule that heat flows from the hotter body to the colder. [5][6][7]
If a physical system is inhomogeneous or very rapidly or irregularly changing (for example, by turbulence), it may be impossible to characterize it by temperature. But still, there can be a transfer of energy as heat between it and another system. If a system has a regular physical state and persists long enough to reach thermal equilibrium with a specified thermometer, it has a temperature according to that thermometer. An empirical thermometer registers the degree of hotness for such a system. Such a temperature is called empirical. [8][9][10] For example, Truesdell writes about classical thermodynamics: At any time, the body has a real number called the temperature. This number is a measure of how hot the body is.[11]
Physical systems that are too turbulent to have temperatures may still differ in hotness. A physical system that passes heat to another physical system is said to be the hottest of the two.
How does a greenhouse work?
Seeds sprout and grow in warm conditions. Greenhouse is a real solution.
- Sunlight passes through the plastic covers of the greenhouse and warms the seed and soil, which radiates the heat back out as infrared radiation.
- These rays can not pass through the plastic.
- Hence, heat is trapped inside, and the temperature rises in the greenhouse.
How does a vacuum flask work?
A vacuum flask keeps the drink hot or cold by stopping heat transfer.
- The flask has two walls and a vacuum in between them.
- Conduction happens only through matter.
- The shining walls reflect the radiation, and the top of the flask is tightly closed by an insulating lid.
Zeroth law of thermodynamics
The Zeroth law of thermodynamics says that no heat transfer occurs between two objects in thermal equilibrium; therefore, they are at the same temperature.
We can calculate the heat released or absorbed using the specific heat capacity C, the mass of the substance m, and the change in temperature Delta T in the equation:
q = m ×C× ∆T
Scientists define heat as thermal energy transferred between two systems at different temperatures that are in contact.
Points to ponder:
- The change in temperature resulting from heat transferred to a system depends on how many molecules are in the system.
- On an atomic level, the molecules in an object are constantly in motion and colliding with each other.
- Every time molecules collide, there is a transfer of kinetic energy.
- When the two systems are in contact, the transfer of heat through molecular collisions from the hot to the cold system.
- The thermal energy flows from the hot to the cold object until the two objects are at the same temperature.
- When the two systems in contact are at the same temperature, we say they are in thermal equilibrium.
Heat capacity
- Heat capacity is a physical property of a substance, i.e., it depends on the state and properties of the substance under consideration.
Before the development of the laws of thermodynamics, heat measurement was by measuring changes in the states of the participating bodies.
Some general rules, with important exceptions, can be stated as follows.
- In general, most bodies expand on heating. In this circumstance, heating a body at a constant volume increases the pressure it exerts on its constraining walls while heating at a constant pressure increases its volume.
Calculation of q using the heat capacity
We can use the heat capacity to determine the heat released or absorbed by a material. Note that mass and specific heat capacity can only have positive values, so the sign of q will depend on delta T.
Imagine I have 250 milliliters of hot tea. I want to cool down before I drink it. The tea is currently at 370K, and I want to cool it down to 350K. How much thermal energy has to be transferred from the tea to the surroundings to cool it to the desired temperature?
The hot tea will transfer heat to the surroundings as it cools. Assuming tea is mostly water, in this calculation, I use the density and heat capacity of water. The specific heat capacity of water is 4.18, and the density of water is 1.00. We can calculate the energy transferred in the process of cooling the tea using the following steps:
The given quantities are:
Mass=250 mL
Density =1g/mL
Specific heat capacity =4.18 J/gK
Initial temperature (T1)=370 K
Final temperature (T2)=350
Temperature difference ∆T= T2-T1=-20K.
I calculated the mass of the tea/water mass=volume*density
m=250*1=250 g
Heat transferred from the hot tea using the equation for heat:
Heat transferred q = mass*specific heat*difference in temperature.
q = m ×C× ∆T
q= 250*4.18*-20=-20900J.
Reference
1 Wikipedia
2 Denbigh, K. (1981), p. 9.
19 ^ Callen, p.19
20 ^ Maxwell, J.C. (1871), Chapter III.
22 ^ Watkinson, John (2001). The Art of Digital Audio. Taylor & Francis. ISBN 978-0-240-51587-8.
25 ^ Guggenheim, E.A. (1967) [1949], Thermodynamics. An Advanced Treatment for Chemists and Physicists (fifth ed.), Amsterdam: North-Holland Publishing Company., p. 157: "It is impossible by any procedure, no matter how idealized, to reduce the temperature of any system to zero temperature in a finite number of finite operations."
26 ^ Pippard, A.B. (1957/1966). Elements of Classical Thermodynamics for Advanced Students of Physics, original publication 1957, reprint 1966, Cambridge University Press, Cambridge, page 51: "By no finite series of processes is the absolute zero attainable."
27 ^ Tisza, L. (1966). Generalized Thermodynamics, MIT Press, Cambridge MA, page 96: "It is impossible to reach absolute zero as a result of a finite sequence of operations."
28 ^ Kittel, Charles; Kroemer, Herbert (1980). Thermal Physics (2nd ed.). W.H. Freeman Company. p. Appendix E. ISBN 978-0-7167-1088-2.
















Comments
Post a Comment