How much Do You Know About The Support Of Our Atmosphere To Live Here?
Without the atmosphere, life here would be impossible. Do you know what the atmosphere is? It is a colorless, tasteless, odorless blanket of gases that surrounds the earth. It provides water to drink and air to breathe. It keeps us warm and protects us from the sun's harmful rays. The atmosphere is nearly 700 km deep but has no distinct boundary. It becomes thinner and eventually fades out as it extends to space.
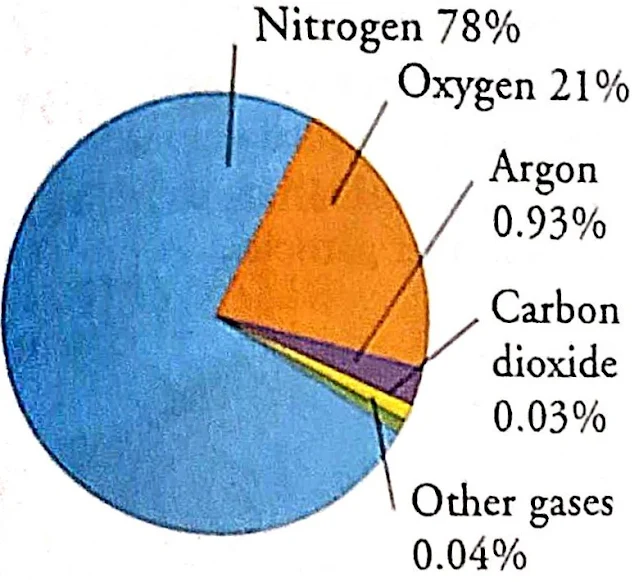
Nitrogen and oxygen cover 99% of the atmosphere (nitrogen 78% and oxygen 21%). It also contains a small amount of argon, carbon dioxide, and traces of other gases. Green plants produce oxygen, which maintains the balance of gases. Air also contains a variable amount of water vapor, around 1% at sea level and 0.4% all over the atmosphere. Air composition, temperature, and pressure vary with altitude.
Earth's early atmosphere consisted of gases in the solar nebula, hydrogen.
The atmosphere changed significantly over time, affected by many factors.
Atmospheric change is due to:
- Volcanism
- Life, and weathering.
- Human activity has also contributed to atmospheric changes, such as global warming, ozone depletion, and acid deposition.
The atmosphere has a mass of about 5.15×1018 kg, three-quarters of which is within about 11 km (6.8 mi; 36,000 ft) of the surface. The atmosphere becomes thinner with increasing altitude, with no definite boundary between the atmosphere and outer space.
Layers of the atmosphere:
There are five layers in the atmosphere. The composition of gases varies within layers.
They are:
- Troposphere, 0 to 12 km
- Stratosphere, 12 to 50 km
- Mesosphere, 50 to 80 km
- Thermosphere, 80 to 700 km
- Exosphere, 700 to 10,000 km
Troposphere:
The troposphere extends up to 12 km above ground level.
Significant features:
- Living things can survive naturally
- Contains 75% of atmospheric gases
- Water vapor present
- Clouds exist
- The temperature drops with height
- Changes in this layer, create weather.
Stratosphere features:
- Contains 19% of atmospheric gases
- Little water vapour
- Very calm
- Temperature rises with height.
Mesosphere features:
- Gases are thin
- The temperature drops rapidly with altitude (less than -110o C)
- Air is still thick air to slow down meteorites.
Thermosphere features:
- Gases are thin
- Absorbs UV rays from the sun
- The temperature rises with altitude (reaching 2000°C).
- The ionosphere is a layer within this layer where ionized gases are present.
- These ionized gases bounce off radio signals.
Exosphere features:
- The outer layer of the atmosphere
- Light gases drifting into space from here.
- The exosphere no longer behaves like a gas
- The particles constantly escape into space.
- The exosphere contains many of the artificial satellites that orbit Earth.
Other Layers
Ionosphere:
- The ionosphere is a part of the mesosphere where ionization by solar radiation happens.
- It is responsible for auroras.
- During the daytime, it stretches from 50 to 1,000 km and includes the mesosphere, thermosphere, and parts of the exosphere.
Homosphere:
The homosphere and heterosphere are defined by whether the atmospheric gases are well-mixed by turbulence.
- The surface-based homosphere includes the troposphere, stratosphere, mesosphere, and the lowest part of the thermosphere. [2]
- This relatively homogeneous layer ends at the turbopause at about 100 km, about 20 km above the mesopause.
- Above this altitude lies the heterosphere, which includes the exosphere and most of the thermosphere.
- Here, the chemical composition varies with height.
Planetary boundary layer:
- The planetary boundary layer is the part of the troposphere closest to Earth's surface and is directly affected by it, mainly through turbulent diffusion.
- During the daytime, the planetary boundary layer is well-mixed, while at night, it becomes stably stratified with weak or intermittent mixing.
- The depth of the planetary boundary layer ranges from as little as about 100 meters on clear, calm nights to 3,000 m or more during the afternoon in dry regions.
Ozone
- The ozone layer is within the stratosphere.
- In this layer, ozone concentrations are about 2 to 8 parts per million, which is much higher than in the lower atmosphere but still very small compared to the main components of the atmosphere.
- It is mainly located in the lower portion of the stratosphere from about 15–35 km, though the thickness varies seasonally and geographically.
- About 90% of the ozone in Earth's atmosphere is in the stratosphere.
This thin layer in the stratosphere protects us from harmful solar radiation (UV radiation). There is depletion in the ozone layer because of chlorofluorocarbons (CFCs). Holes in the ozone layer appear every spring over the poles.
Greenhouse effect:
The greenhouse effect is directly related to this absorption and emission effect. Some gases in the atmosphere absorb and emit infrared radiation but do not interact with light in the visible spectrum (CO2 and H2O are examples).
- Carbon dioxide and other gases have a significant role in trapping the sun's heat.
- They act like glass in a greenhouse.
- This trapping of the sun's heat keeps the earth warm.
Oxygen cycle:
There is a continuous circulation of gases between the atmosphere and living things.
- Animals breathe in oxygen and release carbon dioxide.
- Plants absorb carbon dioxide and release oxygen.
Pressure:
The average atmospheric pressure at sea level is 760.00 mmHg. The total atmospheric mass is 5.1480×1018 kg. Atmospheric pressure is the total weight of the air above the unit area at the point where the pressure is measured. Thus, pressure varies with location and weather.
If the entire mass of the atmosphere had a uniform density equal to sea level density (about 1.2 kg per m3) from sea level upwards, it would terminate abruptly at an altitude of 8.50 km.
Air pressure decreases exponentially with altitude, dropping by half every 5.6 km or by a factor of 1/e (0.368) every 7.64 km (the scale height) for altitudes around 70 km. Over 100 km height, the atmosphere may no longer be well mixed. There, each chemical species has its scale height.
In summary, the distribution of Earth's atmosphere mass is as follows:[3]
· 50% is below 5.6 km (18,000 ft),
· 90% is below 16 km (52,000 ft), and
· 99.99997% is below 100 km (62 mi; 330,000 ft), the Karman line. By international convention, this marks the beginning of space, where human travelers are considered astronauts.
By comparison, Mount Everest is at 8,848 m, and commercial airliners typically cruise between 10 and 13 km, where the lower density and temperature improve fuel economy. Weather balloons reached 30.4 km and above, and the highest X-15 flight in 1963 reached 108.0 km.
Even above the Karman line, significant atmospheric effects such as auroras still occur. Meteors begin to glow in this region, though the larger ones may not burn up until they penetrate more deeply. The various layers of Earth's ionosphere (for HF radio propagation) begin below 100 km and extend beyond 500 km. By comparison, the International Space Station and Space Shuttle typically orbit 350–400 km within the F-layer of the ionosphere, where they encounter enough atmospheric drag to require reboots every few months. Otherwise, orbital decay will occur, and they have to return to Earth. Depending on solar activity, satellites can experience noticeable atmospheric drag at altitudes as high as 700–800 km.
Temperature:
Temperature decreases with altitude starting at sea level, but there are variations. Above 11 km, the temperature stabilizes throughout the rest of the troposphere. In the stratosphere, starting above about 20 km, the temperature increases with altitude due to heating within the ozone layer caused by the absorption of ultraviolet radiation from the Sun. Another region of increasing temperature with height occurs in the thermosphere above 90 km.
Speed of sound:
In an ideal gas of constant composition, the speed of sound depends only on temperature.
The atmospheric pressure or density does not influence the speed of sound.
Density
The density of air at sea level is about 1.2 kg/m3.
We do not measure atmospheric density directly, but we can calculate it from temperature, pressure, and humidity using the equation of state for air (a form of the ideal gas law). Atmospheric density decreases as altitude increases.
Optical properties:
Solar radiation (or sunlight) is the energy that Earth receives from the Sun. Earth also emits radiation back into space, but at longer wavelengths that humans cannot see. The atmosphere absorbs or reflects some incoming and emitted radiation [4][5].
Scattering:
When light passes through Earth's atmosphere, photons interact with it through scattering.
If the light does not interact with the atmosphere, it is called direct radiation and is what you see when looking directly at the Sun.
The scattered light in the atmosphere is indirect radiation.
For example, you cannot see your shadow on an overcast day because no direct radiation reaches you on a cloudy day.
All radiations are scattered.
As another example, due to Rayleigh scattering, shorter (blue) wavelengths scatter more easily than longer (red) wavelengths.
That is the reason why the sky looks blue and the sunset red. During sunset, the Sun is close to the horizon, and the Sun's rays travel through a longer atmospheric distance before reaching your eye. Much of the blue light is scattered away, leaving the red light in a sunset.
Absorption:
Different molecules absorb different wavelengths of radiation. For example, O2 and O3 absorb almost all radiation with wavelengths shorter than 300 nanometres. Water (H2O) absorbs at many wavelengths above 700 nm. When a molecule absorbs a photon, it causes an increase in the energy of the molecule and heats the atmosphere. But the atmosphere also cools by emitting radiation, as discussed below.
The combined absorption spectra of the gases in the atmosphere leave windows of low opacity, allowing the transmission of only certain bands of light. The optical window runs from around 300 nm (ultraviolet-C), the range is roughly 400–700 nm, and continues to the infrared region around 1100 nm. Infrared and radio windows transmit some infrared and radio waves at longer wavelengths. For example, the radio window runs from about one-centimeter to eleven-meter wavelengths.
Emission is the opposite of absorption. Objects tend to emit wavelengths of radiation depending on their black body emission curves. [6]
Now, it is clear that hot objects emit short wavelengths, and cold objects emit long wavelengths. For example, the Sun is approximately 6,000 K (5,730 °C; 10,340 °F), its radiation peaks near 500 nm, and is visible to the human eye. Earth is 290 K (17 °C; 62 °F), so its radiation peaks near 10,000 nm and is invisible. [7]
Because of its temperature, the atmosphere emits infrared radiation. For example, on cloudless nights, Earth's surface cools down faster than on cloudy nights because clouds (H2O) are strong absorbers and emitters of infrared radiation. That is the reason why nights at higher elevations are colder.
References:
1. Anne Marie Helmenstine, PhD (June 16, 2018). "The 4 Most Abundant Gases in Earth's Atmosphere".
2 ^ "homosphere – AMS Glossary". Amsglossary.allenpress.com. Archived from the original on 14 September 2010. Retrieved 2010-10-16.
3 Lutgens, Frederick K. and Edward J. Tarbuck (1995) The Atmosphere, Prentice Hall, 6th ed., pp. 14–17, ISBN 0-13-350612-6
4 "Absorption/reflection of sunlight". Understanding Global Change. Retrieved 2023-06-13.
5 ^ "The Atmospheric Window". National Oceanic and Atmospheric Administration. Retrieved 2023-06-13.
6 Wikipedia
7 Encyclopedia












Comments
Post a Comment